Environmental Monitoring Software
Our enVigil software suite is purpose-built for facility and environmental monitoring, developed from over 35 years of experience delivering EU-GMP compliant solutions.
Designed for regulated environments, enVigil provides full oversight of critical parameters including airborne particulates, differential pressure, temperature, humidity, and airflow. It also supports equipment monitoring for fridges, freezers, and incubators, all within a single, validated platform.
From aseptic filling lines and ATMP cleanrooms to hospital pharmacies and IVF clinics, enVigil systems are deployed worldwide to support compliant, real-time monitoring in high-stakes environments.
At its core, enVigil is built around the latest regulatory standards: EU-GMP Annex 1, cGMP, GAMP, and 21 CFR Part 11. Whether you’re upgrading legacy systems or designing a new facility, we help you move towards compliance and stay there with confidence.
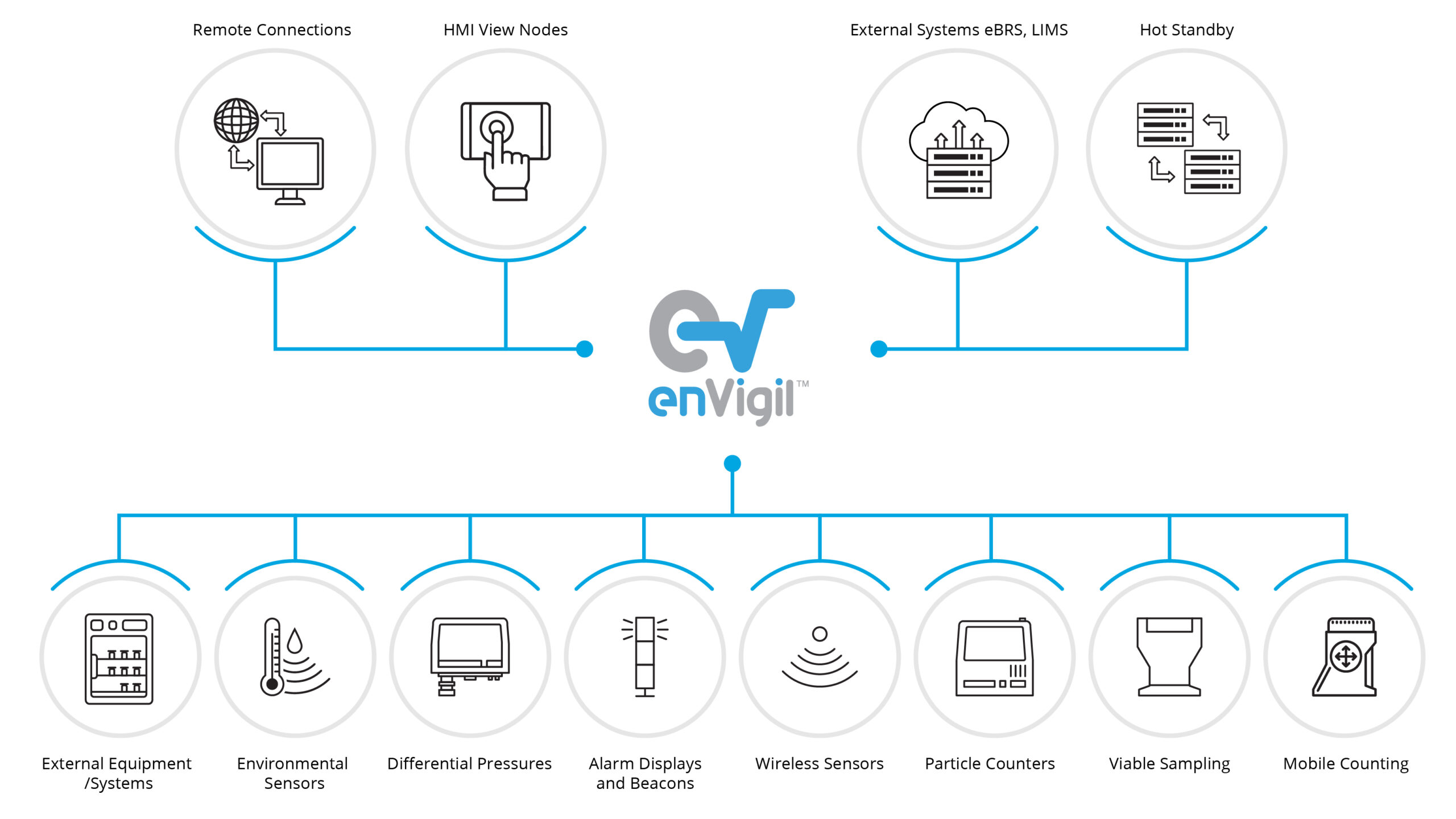
enVigil–V FMS: Environmental Monitoring System
enVigil V offers a configurable environmental monitoring software system aimed at Pharmaceutical, Healthcare, Electronic and Satellite production facilities. enVigil V has evolved from many years of applications with a need to address the regulatory requirements of EU-GMP, cGMP, GAMP and associated standards.
- enVigil V offers support for multiple particle counters, active air samplers and environmental sensors
- Modern dashboard interface gives ability to show high level stats and system parameters
- Server clusters support multiple network clients sharing information
- Cloud connectivity enables data to be sent to third party applications
- SQL Data to third party databases
- Multi-level mimic diagrams
- Trend displays with multiple cursors
- Reconciled alarm reports with Trend Metrics
- Touch screen clients, Tablets, Alarm message displays and beacons
- Interfaces to external systems via OPC, ModBus and ODBC/SQL database applications
- SMS text and email alerts
- Server/Client architecture with network clients
- Hot standby functionality (optional)
- Batch reporting with electronic signatures
- Installed on Windows 10, Windows Server and virtual machine Windows OS platforms
enVigil V is available as both development and runtime licenses with license sizes of 25, 50, 100, 200, 500 and 10,000 Input/ Output (I/O) point database. Up to 128 individual tasks can run concurrently within the enVigil V application and these can range from individual I/O drivers, SMS text /email communications, auto-archiving, real time calculations, scheduling, batch reporting and ModBus/ OPC interfaces
21 CFR Part 11
Compliance Assured
Pharmagraph software has been written from the ground up to comply with the tough demands of 21 CFR part 11 through the use of tight security, proprietary binary logging and provision of an audit trail. The software system is secured via unique usernames and passwords, with automatic log out when the system is left idle. A secure binary format is used for data log files making it virtually impossible to alter records. An audit trail logger automatically tracks changes to the system configuration, recording what has been changed, by whom, when and for what reason. The audit trail logger also records operator log in and log out activity, including failed log in attempts. Our aim is to create a complete package that helps our customers to become more compliant in less time.

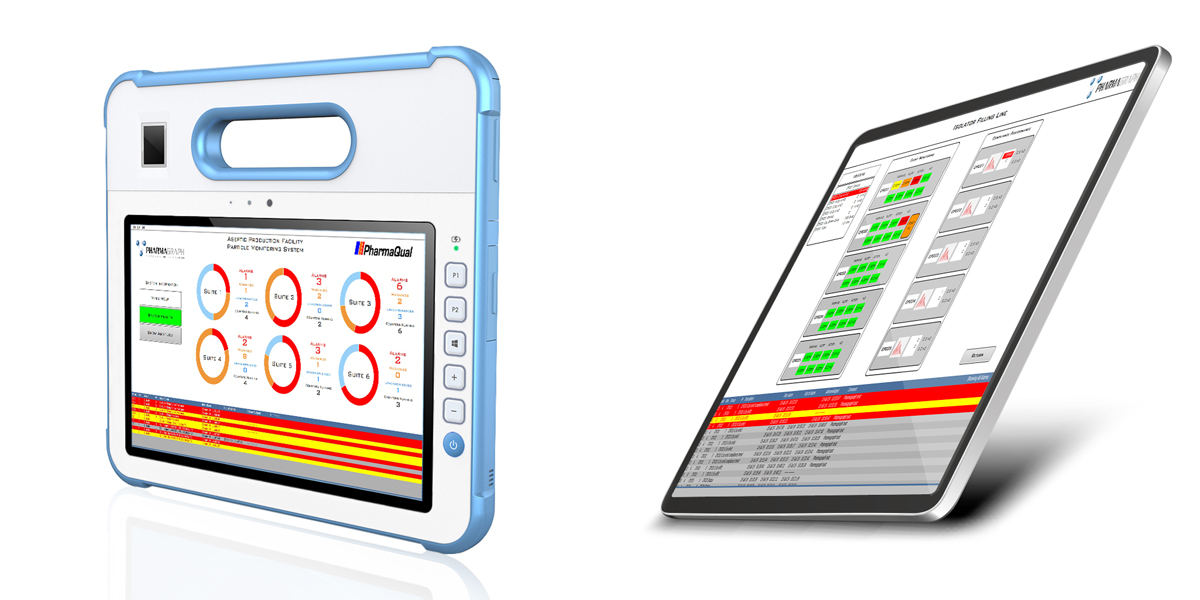
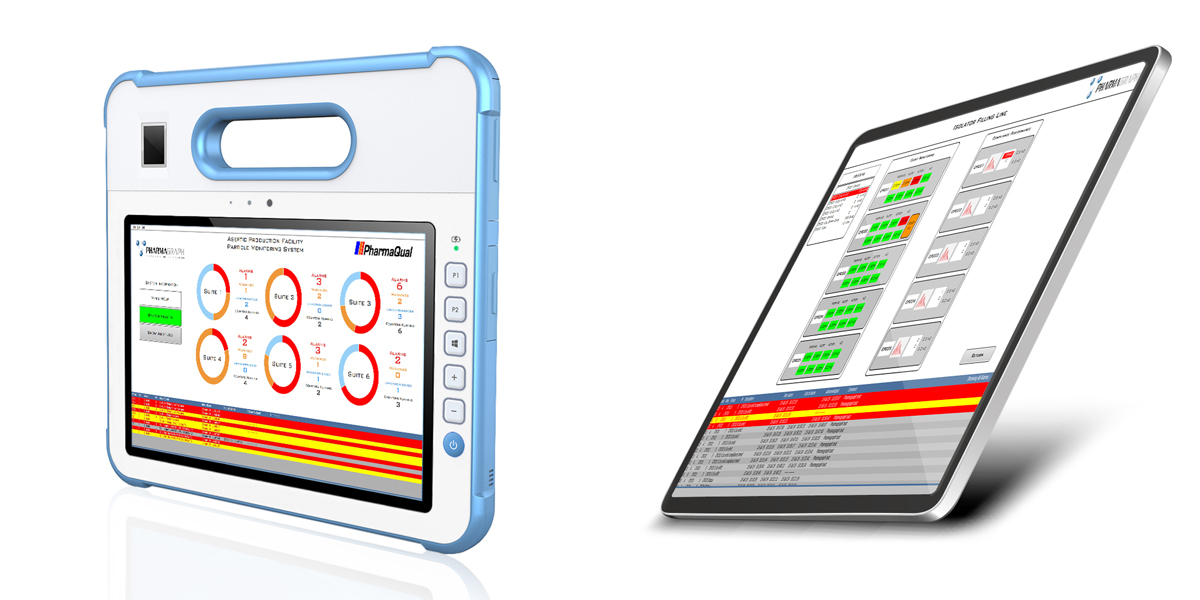
Standard and medical tablet deployment
enVigil–IMS: Isolator Monitoring System
Particle Monitoring for Isolators and RABS Filling Machines
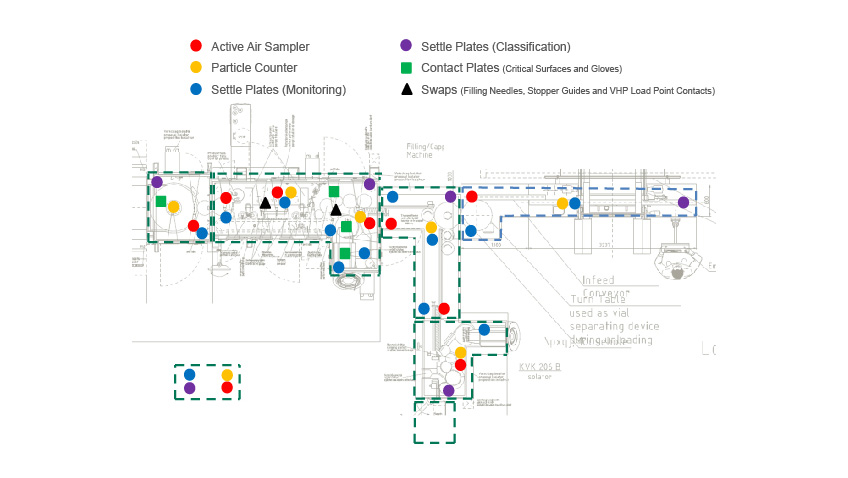
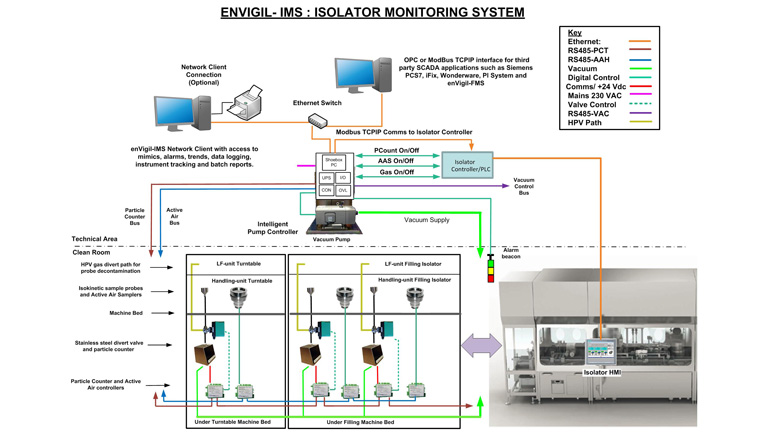
Isolator and filling machine manufacturers provide both bespoke isolator filling machines for specific products and the new multi format isolator for multiple products. Pharmagraph have developed the enVigil-IMS Isolator Monitoring System to address this emerging technology. enVigil-IMS is provided with an overview menu with status indicators, trends, alarms, data logging, instrument tracking, batch reports and OPC data to third party SCADA applications. Optional modules are also available to support network view nodes, electronic signatures and an ODBC/SQL Database exchange utility to collect logged data from the enVigil-IMS application. enVigil- IMS offers support for isolator gassing operations and particle counter “swap-ability” in order to minimise system downtime due to contamination failures. The intelligent pump controller (P5050) supports up to eight particle counters with a central vacuum pump and up to eight active air samplers and includes a digital control interface to the Isolator controller PLC. enVigil-IMS is complete with an application specific GAMP compliant documentation suite that includes a Functional Design Specification and Installation and Operational Acceptance Test Protocols allowing the enVigil-IMS system to be easily designed, configured, installed, commissioned and verified.
Scalable, modular solution
The enVigil-IMS application allows configuration of the system to include sampling point descriptors, alert (warning) and action (alarm) levels, counts per cubic feet and counts per cubic metre for >0.5μm and >5.0μm size channels, hydrogen peroxide protection valves and instrument tracking facilities. The enVigil-IMS application provides a data historian function maintaining historic information for alarms, periodic data and audit logs, all of which are maintained in a 21 CFR Part 11 compliant environment.
Particle Counting
enVigil-IMS offers particle counting with Hydrogen Peroxide protection for Isolator applications:
Pharmagraph S1816VS
For applications with a remote vacuum source and offering “Swap-ability” in the clean room without the need for re-programming and thereby reducing system down time.
Stainless Steel Diverter Ball Valve
To protect the particle counter from the ingress of Hydrogen Peroxide Vapour and condensing deposits. These occur during decontamination and which over time will render the particle counter useless.
Integral Viable Monitoring
The Pharmagraph air sampler provides an integral fan impeller which is monitored and controlled to maintain the correct sample volume and D50 value. The active air sampler is provided in 316 grade stainless steel and can be provided with a conventional screw head or a 1/16 turn bayonet fitted head with either a pedestal base or tri-clamp mount.
21 CFR Part 11
Compliance Assured
Pharmagraph software has been written from the ground up to comply with the tough demands of 21 CFR part 11 through the use of tight security, proprietary binary logging and provision of an audit trail. The software system is secured via unique usernames and passwords, with automatic log out when the system is left idle. A secure binary format is used for data log files making it virtually impossible to alter records. An audit trail logger automatically tracks changes to the system configuration, recording what has been changed, by whom, when and for what reason. The audit trail logger also records operator log in and log out activity, including failed log in attempts. Our aim is to create a complete package that helps our customers to become more compliant in less time.

enVigil–PnP: Monitoring System
Demonstrates GMP/GLP compliance for cleanrooms and laboratories.
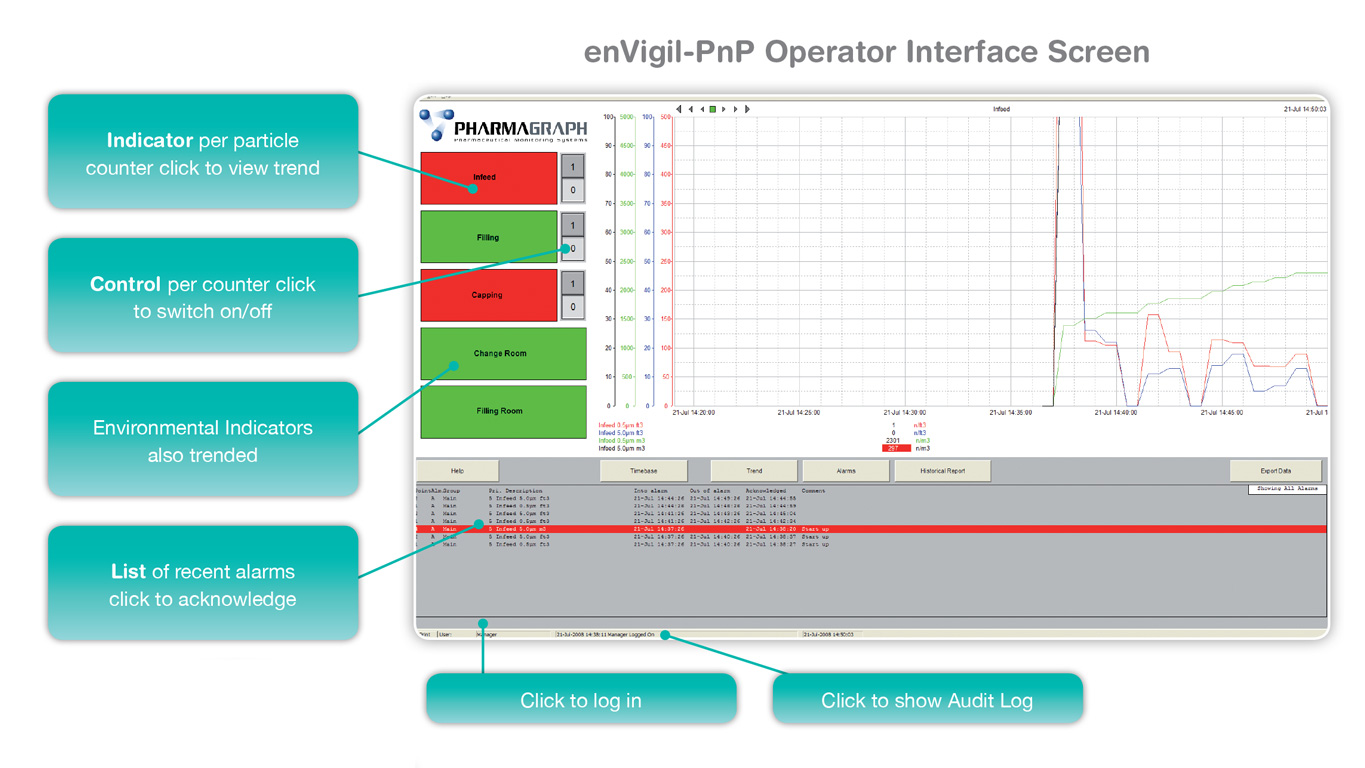
enVigil-PnP provides a simple to use, cost effective solution which ensures a GMP/GLP compliant system and is ideally suited to modern day hospital pharmacy and IVF clinic operations. With a range of package sizes supporting up to 20 particle counters, 100 active air samplers and up to 160 environmental inputs enVigil-PnP is easy to deploy, verify and validate.
enVigil-PnP is provided with an overview menu with status indicators, trends, alarms, data logging, reports and data to MS Excel. Optional modules are also available to support instrument tracking, batch reporting, network view nodes, OPC server and GSM modem support for data on alarm to email and SMS text.
enVigil-PnP offers support for isolator gassing operations and particle counter “swap-ability” in order to minimise system downtime due to contamination failures. If you do not require particle counting, then enVigil-PnP offers a low-cost version for environmental or viable (active air sampling) monitoring only.
enVigil-PnP is complete with a simple Verification Protocol that includes a Functional Design Specification and Installation and Operational Acceptance Test Protocols allowing the enVigil-PnP system to be easily designed, configured, installed, commissioned and verified.
Scalable, modular solution
Pharmagraph’s monitoring systems can start small, saving initial investment, then scale up as the facility grows, building on existing hardware and monitoring sensors and providing additional monitoring stations as the system grows. Initial systems can start as small as a single environmental parameter and scale up to an entire facility of parameters because of its modular nature
Particle Counting
enVigil-PnP supports two particle counters detailed as follows:
Pharmagraph S1816 Series Particle Counter
The Pharmagraph S1816 series particle counter contains a Met One 6015 sensor and is housed in a stainless-steel enclosure suitable for pharmaceutical applications. The S1816 particle counter offers either Ethernet or RS485 Serial communication protocols. The Met One 6015 sensor is compliant with the ISO 21501-4 calibration standard for particle counters. The Met One 6015 sensor provides >0.5μm and >5.0μm size channels at a 1.0 cfm (28.3 L/min) flow rate. The S1816VS serial version offers “swap-ability” when deployed with Pharmagraph vacuum pump controllers (P4150-4G/CC9058). This aids quick replacement if the sensor requires a calibration service or becomes contaminated, as the particle counter network address is determined by the sampling position and not the individual sensor. This in turn reduces system downtime.
Met-One 6015P Particle Counter
The Met-One 6015P particle counter offers a particle counter with an integrated vacuum pump and is compliant with the ISO 21501- 4 calibration standard for particle counters. The 6015P provides >0.5μm and >5.0μm size channels at a 1.0 cfm (28.3 L/min) flow rate and offers Ethernet connectivity. The Met-One 6015P provides an integrated pump particle counter solution which reduces installation costs and can easily be fitted to Isolators, Micro Biological Safety Cabinets and LAF workstations.

Building Blocks for Compliance
Pharmagraph’s unique approach enables you to deploy a compliant monitoring system with minimal configuration and documentation by using standard building blocks. With the introduction of the highly capable enVigil PnP offering, you can now put together a system more rapidly than ever.
How it works
enVigil PnP software uses a click-to-configure software system and a template approach to qualification documentation. Highly functional hardware building blocks map directly onto equivalent software configuration screens and document templates.
The building blocks cover three categories to suit the scope of your application:
- Airborne particle monitoring
- Environmental monitoring
- Microbiological monitoring
21 CFR Part 11
Compliance Assured
Pharmagraph software has been written from the ground up to comply with the tough demands of 21 CFR part 11 through the use of tight security, proprietary binary logging and provision of an audit trail. The software system is secured via unique usernames and passwords, with automatic log out when the system is left idle. A secure binary format is used for data log files making it virtually impossible to alter records. An audit trail logger automatically tracks changes to the system configuration, recording what has been changed, by whom, when and for what reason. The audit trail logger also records operator log in and log out activity, including failed log in attempts. Our aim is to create a complete package that helps our customers to become more compliant in less time.
Standard and medical tablet deployment

The team at Pharmagraph made integration seamless. The system passed validation with no findings and has been faultless since.”
Environmental Monitoring Lead, Biotech Facility
Who We Are. What We Deliver.
From decades of industry experience to the systems we build today, everything we do is grounded in compliance, performance, and trust. Join us behind the scenes of world-class pharmaceutical monitoring, and help shape the solutions that keep our partners ahead.
Accreditations & Assurance
Our pharmaceutical compliance software meets the latest regulatory expectations, including 21 CFR Part 11 and GAMP 5. We build confidence into every system, but we back it with proof.