Software
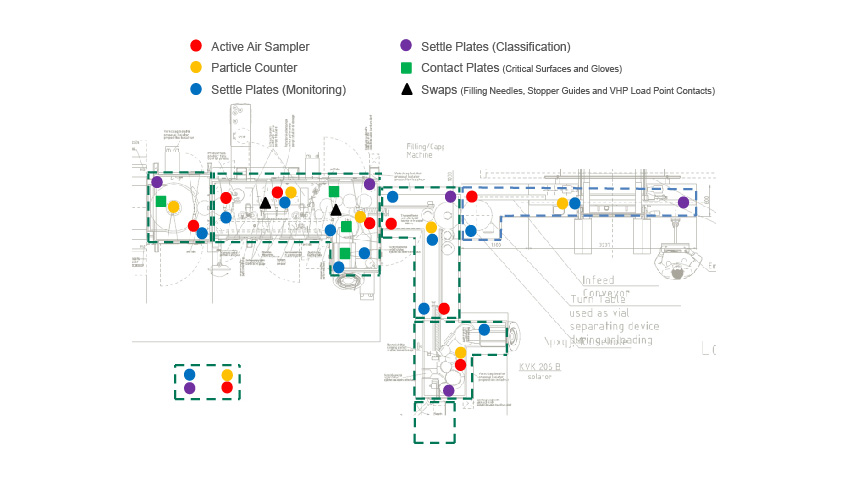
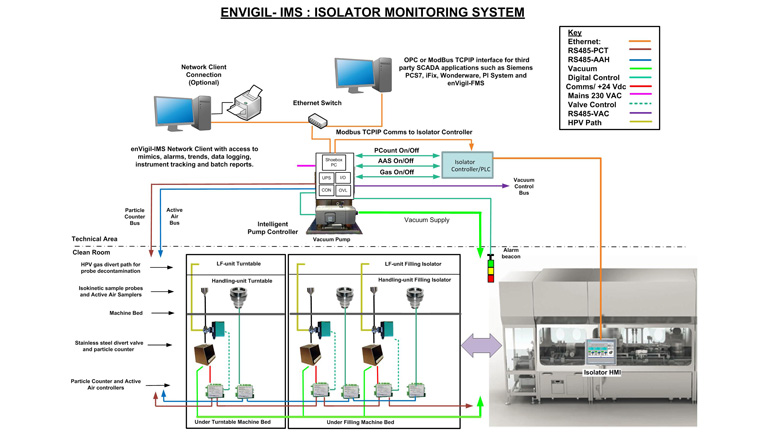
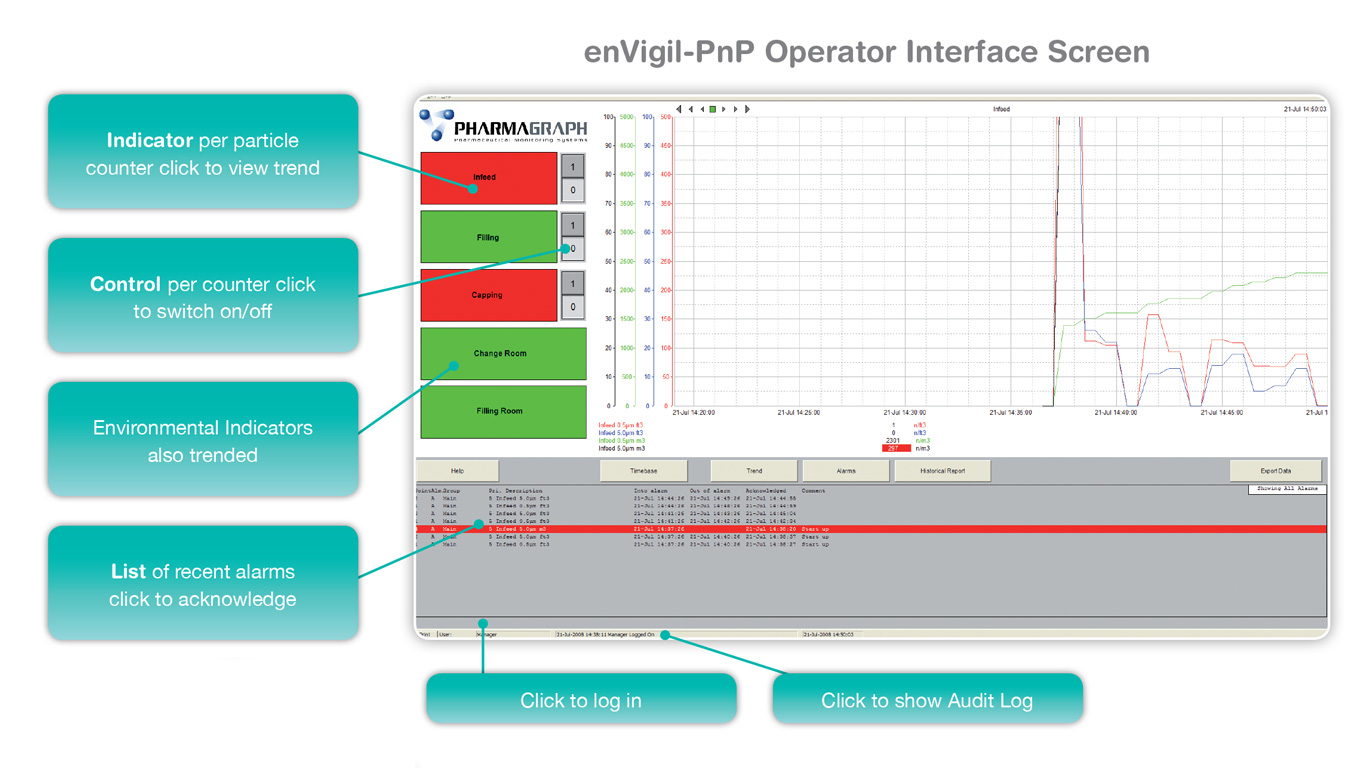
Pharmagraph publish the enVigil software suite which is deployed specifically for facility and environmental monitoring applications. With a pedigree that has been derived from thirty five years’ experience in providing EU-GMP compliant monitoring solutions, the enVigil software range offers an EU-GMP/GLP compliant monitoring system for a range of applications. From pharmaceutical cleanrooms to satellite manufacturing facilities, Pharmagraph’s enVigil systems are deployed worldwide to measure environmental parameters such as airborne particulates, differential pressure, temperature, humidity and air flow. These systems may also be expanded to include equipment monitoring of fridges, freezers and incubators. The monitored facilities range from pharmaceutical aseptic filling suites, stem cell/ATMP facilities, IVF clinics, hospital pharmacies, blood banks, cold chain storage suites and satellite manufacturing facilities.
At the heart of the enVigil suite of software is the adherence to the requirements of the EU-GMP Annex 1, cGMP, GAMP and 21 CFR Part 11 thereby ensuring the installed system can meet current regulatory requirements. We specialise in helping our customers to move towards compliance.
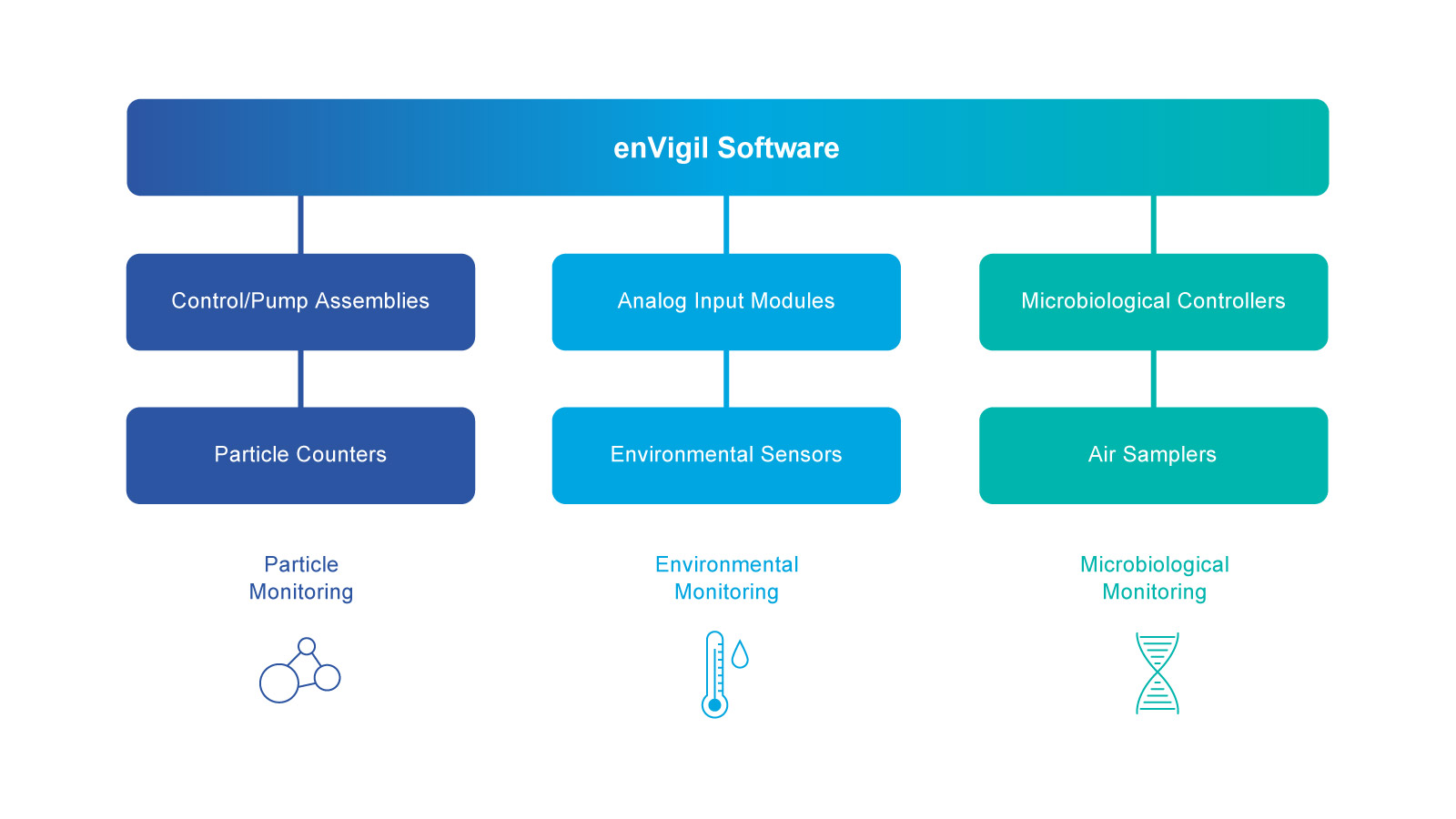
The enVigil suite of software is provided in three variants: